Cold Plasma, Hot Potential: A New Frontier in Wound Healing from the Netherlands
- Nikki Johnston
- Jul 14, 2025
- 2 min read
Chronic wounds have long challenged both clinicians and healthcare systems. With an aging population, rising rates of diabetes, and increasing care fragmentation, the demand for effective, efficient wound therapies has never been greater.
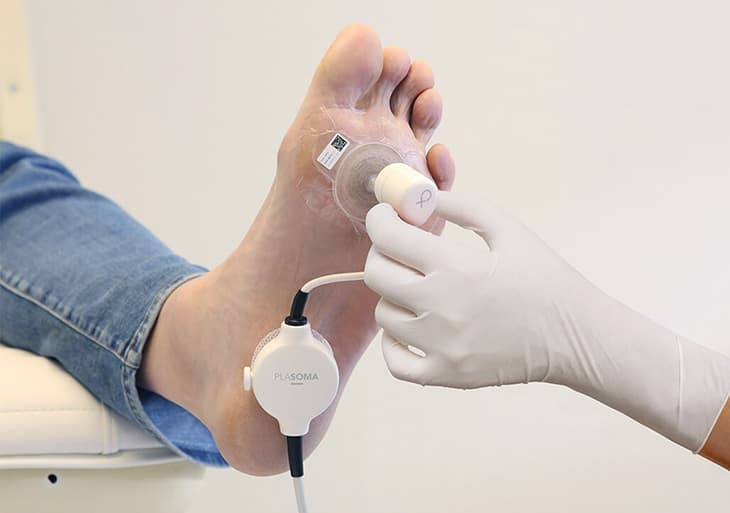
Enter Plasmacure, a Dutch MedTech company making waves with their PLASOMA cold plasma system-a novel approach to wound healing that just secured €6 million in Series A funding to expand its reach.
What is Cold Plasma in Wound Care?
Cold plasma, or non-thermal plasma, is a partially ionized gas that can be safely applied to living tissue. It works by delivering controlled energy to the wound site, which can:
Reduce bacterial load, even in biofilm-heavy wounds
Stimulate microcirculation
Accelerate healing at the cellular level
Reduce the need for systemic antibiotics
Plasmacure’s PLASOMA device delivers this therapy through a disposable wound patch and a reusable cold plasma generator. It is CE-marked in Europe and has shown early promise in the treatment of hard-to-heal diabetic foot and pressure ulcers.
Who Is Currently Using Cold Plasma?
Cold plasma wound care is already in use, particularly in Europe, where regulatory pathways are more established for emerging therapies.
Germany and the Netherlands have seen pilot deployments in both inpatient and outpatient settings.
Leipzig University Hospital (Germany) has integrated cold plasma into chronic wound protocols.
Radboud University Medical Center (Netherlands) has studied the effect of plasma therapy on wound microbiota and healing rates.
Other companies in Europe, such as Neoplas Tools and Adtec Healthcare, are also actively using and testing cold plasma devices.
In the United States, cold plasma is currently used in research environments and clinical trials, including:
Thomas Jefferson University (Philadelphia), which has conducted preclinical work exploring cold plasma's impact on chronic wounds
Several academic and hospital-based programs exploring its potential for burn wounds, diabetic ulcers, and infected surgical sites
FDA approval is still limited for wound-specific use in the U.S., so widespread clinical adoption is pending further data and regulatory review.
Why This Matters
This innovation represents more than a new device. It reflects a larger shift in wound care:
A move toward non-invasive, tech-enabled therapies
Greater emphasis on biofilm control and bacterial management
A demand for therapies that accelerate healing and reduce hospitalizations
Cold plasma’s ability to reduce microbial load without damaging tissue holds significant potential in managing complex wounds, especially in populations where antibiotics are overused or healing is delayed.
Innovation in wound care often happens quietly, but its impact can be profound. As devices like PLASOMA move through clinical trials and market expansion, cold plasma could become a transformative option for chronic wounds that resist conventional treatment.
With careful evaluation, regulatory support, and clinical evidence, cold plasma may soon shift from emerging tech to everyday tool in the fight against hard-to-heal wounds.



Comments